- Electronegative Elements Chart
- Electronegative Elements Periodic Table
- Electronegative Elements On The Periodic Table
- Electronegative Elements
Thus, fluorine is the most electronegative element, while francium is one of the least electronegative. (Helium, neon, and argon are not listed in the Pauling electronegativity scale, although in the Allred-Rochow scale, helium has the highest electronegativity.). Electronegativity chart of all elements is mentioned below. (Note: Electronegativity has no unit. Linus Pauling was a scientist who designed a scale of electronegative that ranks the elements with respect to each other. And this scale is known as Pauling electronegativity scale.). Electronegativity of Chemical Elements Electronegativity, symbol χ, is a chemical property that describes the tendency of an atom to attract electrons towards this atom. The Pauling electronegativity values of the element ranges from the most electronegative element (Fluorine having electronegativity = 3.98) to least electronegative element (Francium having electronegativity = 0.7) Bonus Gift for you: Interactive Periodic Table Let me tell you how this Interactive Periodic Table will help you in your studies. Interactive periodic table with up-to-date element property data collected from authoritative sources. Look up chemical element names, symbols, atomic masses and other properties, visualize trends, or even test your elements knowledge by playing a periodic table game!
Electronegative Elements Chart
Printable charts below ↓
| NUMBER | SYMBOL | ELEMENT | ELECTRONEGATIVITY |
|---|---|---|---|
| 1 | H | Hydrogen | 2.20 |
| 2 | He | Helium | no data |
| 3 | Li | Lithium | 0.98 |
| 4 | Be | Beryllium | 1.57 |
| 5 | B | Boron | 2.04 |
| 6 | C | Carbon | 2.55 |
| 7 | N | Nitrogen | 3.04 |
| 8 | O | Oxygen | 3.44 |
| 9 | F | Fluorine | 3.98 |
| 10 | Ne | Neon | no data |
| 11 | Na | Sodium | 0.93 |
| 12 | Mg | Magnesium | 1.31 |
| 13 | Al | Aluminum | 1.61 |
| 14 | Si | Silicon | 1.90 |
| 15 | P | Phosphorus | 2.19 |
| 16 | S | Sulfur | 2.58 |
| 17 | Cl | Chlorine | 3.16 |
| 18 | Ar | Argon | no data |
| 19 | K | Potassium | 0.82 |
| 20 | Ca | Calcium | 1.00 |
| 21 | Sc | Scandium | 1.36 |
| 22 | Ti | Titanium | 1.54 |
| 23 | V | Vanadium | 1.63 |
| 24 | Cr | Chromium | 1.66 |
| 25 | Mn | Manganese | 1.55 |
| 26 | Fe | Iron | 1.83 |
| 27 | Co | Cobalt | 1.88 |
| 28 | Ni | Nickel | 1.91 |
| 29 | Cu | Copper | 1.90 |
| 30 | Zn | Zinc | 1.65 |
| 31 | Ga | Gallium | 1.81 |
| 32 | Ge | Germanium | 2.01 |
| 33 | As | Arsenic | 2.18 |
| 34 | Se | Selenium | 2.55 |
| 35 | Br | Bromine | 2.96 |
| 36 | Kr | Krypton | 3.00 |
| 37 | Rb | Rubidium | 0.82 |
| 38 | Sr | Strontium | 0.95 |
| 39 | Y | Yttrium | 1.22 |
| 40 | Zr | Zirconium | 1.33 |
| 41 | Nb | Niobium | 1.6 |
| 42 | Mo | Molybdenum | 2.16 |
| 43 | Tc | Technetium | 1.9 |
| 44 | Ru | Ruthenium | 2.2 |
| 45 | Rh | Rhodium | 2.28 |
| 46 | Pd | Palladium | 2.20 |
| 47 | Ag | Silver | 1.93 |
| 48 | Cd | Cadmium | 1.69 |
| 49 | In | Indium | 1.78 |
| 50 | Sn | Tin | 1.96 |
| 51 | Sb | Antimony | 2.05 |
| 52 | Te | Tellurium | 2.1 |
| 53 | I | Iodine | 2.66 |
| 54 | Xe | Xenon | 2.6 |
| 55 | Cs | Cesium | 0.79 |
| 56 | Ba | Barium | 0.89 |
| 57 | La | Lanthanum | 1.10 |
| 58 | Ce | Cerium | 1.12 |
| 59 | Pr | Praseodymium | 1.13 |
| 60 | Nd | Neodymium | 1.14 |
| 61 | Pm | Promethium | 1.13 |
| 62 | Sm | Samarium | 1.17 |
| 63 | Eu | Europium | 1.2 |
| 64 | Gd | Gadolinium | 1.2 |
| 65 | Tb | Terbium | 1.22 |
| 66 | Dy | Dysprosium | 1.23 |
| 67 | Ho | Holmium | 1.24 |
| 68 | Er | Erbium | 1.24 |
| 69 | Tm | Thulium | 1.25 |
| 70 | Yb | Ytterbium | 1.1 |
| 71 | Lu | Lutetium | 1.27 |
| 72 | Hf | Hafnium | 1.3 |
| 73 | Ta | Tantalum | 1.5 |
| 74 | W | Tungsten | 2.36 |
| 75 | Re | Rhenium | 1.9 |
| 76 | Os | Osmium | 2.2 |
| 77 | Ir | Iridium | 2.2 |
| 78 | Pt | Platinum | 2.28 |
| 79 | Au | Gold | 2.54 |
| 80 | Hg | Mercury | 2.00 |
| 81 | Tl | Thallium | 1.62 |
| 82 | Pb | Lead | 2.33 |
| 83 | Bi | Bismuth | 2.02 |
| 84 | Po | Polonium | 2.0 |
| 85 | At | Astatine | 2.2 |
| 86 | Rn | Radon | no data |
| 87 | Fr | Francium | 0.7 |
| 88 | Ra | Radium | 0.89 |
| 89 | Ac | Actinium | 1.1 |
| 90 | Th | Thorium | 1.3 |
| 91 | Pa | Protactinium | 1.5 |
| 92 | U | Uranium | 1.38 |
| 93 | Np | Neptunium | 1.36 |
| 94 | Pu | Plutonium | 1.28 |
| 95 | Am | Americium | 1.3 |
| 96 | Cm | Curium | 1.3 |
| 97 | Bk | Berkelium | 1.3 |
| 98 | Cf | Californium | 1.3 |
| 99 | Es | Einsteinium | 1.3 |
| 100 | Fm | Fermium | 1.3 |
| 101 | Md | Mendelevium | 1.3 |
| 102 | No | Nobelium | 1.3 |
| 103 | Lr | Lawrencium | no data |
| 104 | Rf | Rutherfordium | no data |
| 105 | Db | Dubnium | no data |
| 106 | Sg | Seaborgium | no data |
| 107 | Bh | Bohrium | no data |
| 108 | Hs | Hassium | no data |
| 109 | Mt | Meitnerium | no data |
| 110 | Ds | Darmstadtium | no data |
| 111 | Rg | Roentgenium | no data |
| 112 | Cn | Copernicium | no data |
| 113 | Uut | Ununtrium | no data |
| 114 | Fl | Flerovium | no data |
| 115 | Uup | Ununpentium | no data |
| 116 | Lv | Livermorium | no data |
| 117 | Uus | Ununseptium | no data |
| 118 | Uuo | Ununoctium | no data |
The table of electronegativity values has been calculated from a knowledge of the element's ionisation energy and electron affinity. |
Definition

The electronegativity of an element is a measure of its ability to attract electrons from within a covalent bond.
It is an artificial scale from 0.7 to 4.0, created by combining the ionisation energy and the electron affinity of the elements. There are several versions of electronegativity although the most commonly used is that developed by Linus Pauling.

- The most electronegative element is fluorine, value = 4.0
- The least electronegative (sometimes called the most electropositive) is caesium, value = 0.7
Electronegative Elements Periodic Table
Only elements with electronegativity values of 3.0 and above are considered electronegative.
Descending a group
The electronegativity decreases on descending a group. This makes common sense as electronegativity is derived from the ionisation energy and electron affinity, which both decrease on descending a group.
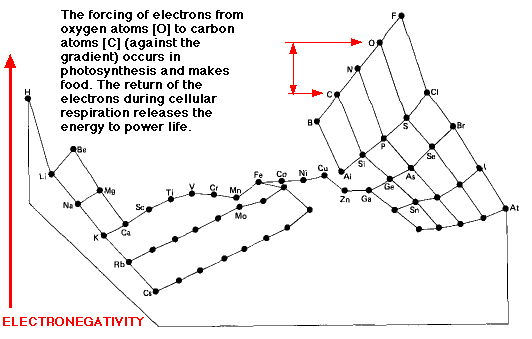
Electronegative Elements On The Periodic Table
Crossing a period
The electronegativity increases from left to right across a period, the most electronegative elements are nitrogen, oxygen and fluorine (in increasing order)
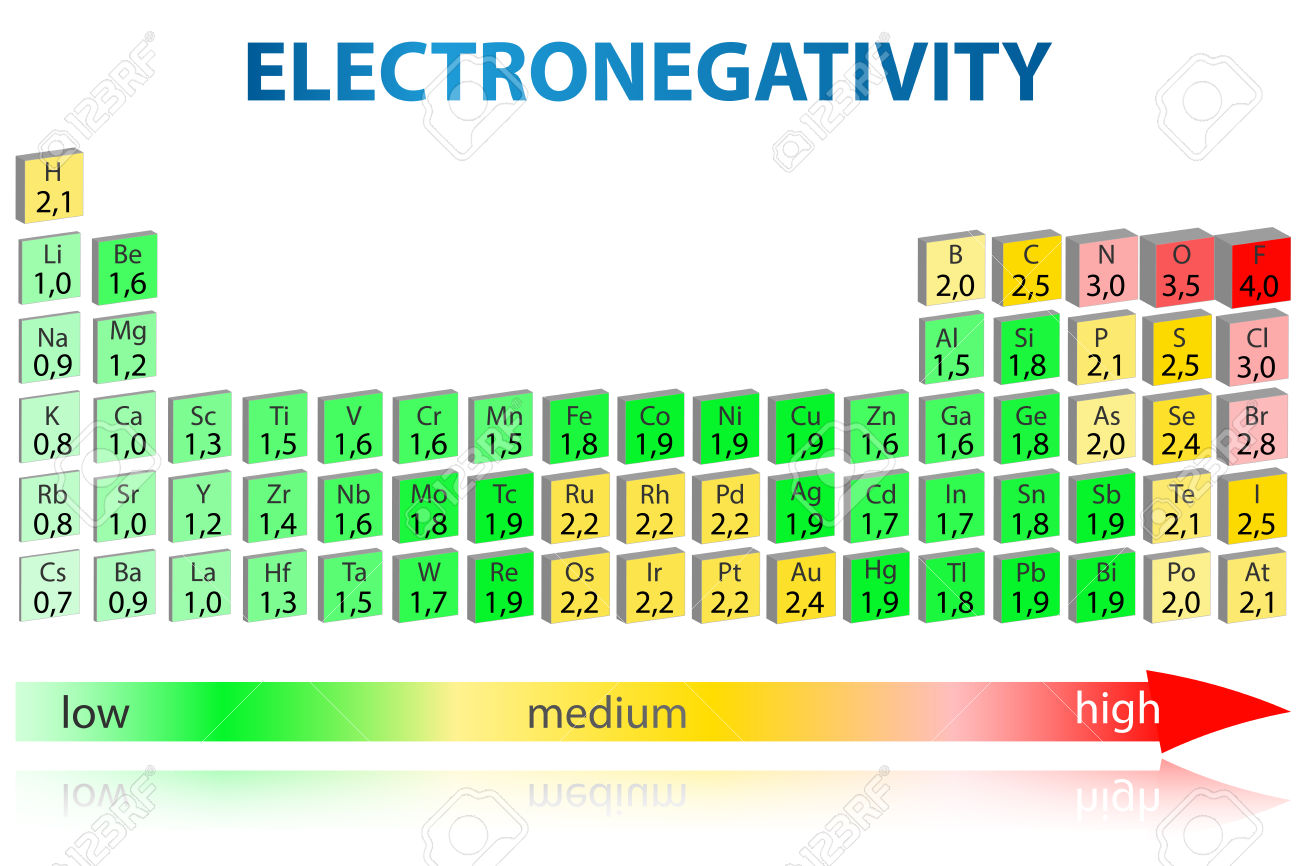
Comparing electronegativities
Information about the nature of chemical bonding can be predicted by looking at the electronegativities of the bonding atoms. The greater the difference in electronegativity the greater the polarisation of the bond.
Carbon has an electronegativity of 2.5 and hydrogen 2.1. These two values are relatively close to one another, so bonds formed between carbon and hydrogen are considered non-polar.
However, bonds formed between oxygen (3.5) and hydrogen (2.1) are highly polarised, (This is the basis behind intermolecular hydrogen bonding) whereas bonds formed between oxygen and elements with a lower electronegativity than hydrogen are polarised to such an extent that they are ionic.
In the same way the nature of the chlorides of the elements may be predicted.
Electronegative Elements
| Aluminium chloride formed from aluminium (electronegativity = 1.5) and chlorine (3.0) falls right on the borderline of ionic/covalency. In fact, aluminium chloride is ionic at low temperatures, but covalent as the temperature rises. We consider it to be a covalent compound with highly polarised bonds. (the molecule itself is non-polar due to symmetry). |
These 'rules' are not empirical in that they cannot be used mathematically. There is no 'magic' difference in electronegativity at which the change between ionic and covalent occurs, if this were the case then metal nitrides and sulfides would not be ionic. It is more a case of getting a feel for the concept and using the electronegativity values as a rough guide.
